Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sakurai, Kiyoshi; Nojiri, Ichiro*
JAERI-Conf 2003-019, p.855 - 857, 2003/10
This paper provides overview of sub-criticality safety analysis seminar (July 2000-July 2003, JAERI, total 40 engineers from universities, research institutes and enterprises) for nuclear fuel cycle facility with the Monte Carlo method in Japan. MCNP-4C2 system (MS-DOS version) was installed in each note-type personal computer. Fundamental theory of reactor physics and Monte Carlo simulation including MCNP-4C manual was lectured. Effective neutron multiplication factor and neutron spectrum were calculated for JCO deposit tank, JNC uranium solution storage tank, JNC plutonium solution storage tank and JAERI TCA core. In the seminar, methodology of safety management for nuclear fuel cycle facility was discussed in order to prevent criticality accident.
Nuclear Non-Proliferation Study Group*; *; Iwata, Shuichiro*; *; *; *;
JNC TN1400 2000-008, 81 Pages, 2000/04
no abstracts in English
Mizuta, Shunji; ;
JNC TN9400 2000-032, 38 Pages, 2000/03
lt is necessary for feasibility study of fast reactor to evaluate the oxidation of the austenitic stainless steels in the case of using for core material in carbon dioxide gas-cooled reactor. The properties for oxidation of austenitic stainless steels in carbon dioxide were surveyed in literatures and the data were selected after evaluation of factors for oxidation in carbon dioxide. The equation of oxidation in carbon dioxide for PE16, 20Cr/25Ni/Nb, 18Cr-8Ni and JNC Cladding materials were proposed. The equation for oxidation of austenitic stainless steels were expressed as upper limit for the equation according to parabolic law. The equation for JNC cladding materials (PNC316, PNC1520, 14Cr-25Ni) was proposed based the oxidation behavior of 18Cr-8Ni which is same oxidation region for weight gain in three-component system of Fe-Cr-Ni, in addition to evaluate of effect for silicon content. The oxidation equation of 20Cr/25Ni/Nb was applied to the high Ni alloy of JNC cladding material. The obtained equation is as follows, X = 4.4W 1000, W =
1000, W =  , kp =
, kp =  exp(-Q/(RT)), X: oxide thickness[
exp(-Q/(RT)), X: oxide thickness[ m], W : weight gain[g
m], W : weight gain[g cm
cm ], kp : parabolic rate constant[g
], kp : parabolic rate constant[g
 cm
cm
 s
s ], t :time[sec]
], t :time[sec]  : constant[g
: constant[g
 cm
cm
 S
S ], Q : activation energy[J・mol
], Q : activation energy[J・mol ], R : gas constant[8.314J
], R : gas constant[8.314J  K
K
 mol
mol ], T : temperature[K] (1) PE16 : kp = 1.090
], T : temperature[K] (1) PE16 : kp = 1.090 10
10 exp(-192,500/(RD)), (2) 20Cr/25Ni/Nb : kp = 1.651
exp(-192,500/(RD)), (2) 20Cr/25Ni/Nb : kp = 1.651 10
10 exp(-201,300/(RT)) High Ni alloy (JNC), (3)18Cr-8Ni : kp = 1.503
exp(-201,300/(RT)) High Ni alloy (JNC), (3)18Cr-8Ni : kp = 1.503 10
10 exp(-60,000/(RT)), (4) PNC316, PNC1520 : kp = 1.503
exp(-60,000/(RT)), (4) PNC316, PNC1520 : kp = 1.503 10
10 exp(-60,000/(RT))
exp(-60,000/(RT)) 0.62
0.62 14Cr-25Ni(JNC) The weight gain is (3)
14Cr-25Ni(JNC) The weight gain is (3)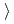 (4)
(4)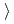 (2)
(2)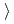 (1) in order.
(1) in order.
Yui, Mikazu; ; Shibata, Masahiro
JNC TN8400 99-070, 106 Pages, 1999/11
This report is a summary of status, frozen datasets, and future tasks of the JNC thermodynamic database (JNC-TDB) for assessing performance of high-level radioactive waste in geological environments. The JNC-TDB development was carried out after the first progress report on geological disposal research in Japan (H3). In the development, thermodynamic data (equilibrium constants at 25  C, I=0) for important radioactive elements were selected/determined based on original experimental data using different models (e.g., SIT, Pitzer). As a result, the reliability and traceability of the data for most of the important elements were improved over those of the PNC-TDB used in H-3 report. For detailed information of data analysis and selections for each element, see the JNC technical reports listed in this document.
C, I=0) for important radioactive elements were selected/determined based on original experimental data using different models (e.g., SIT, Pitzer). As a result, the reliability and traceability of the data for most of the important elements were improved over those of the PNC-TDB used in H-3 report. For detailed information of data analysis and selections for each element, see the JNC technical reports listed in this document.
Oda, Chie; Arthur, R. C,*; Sasamoto, Hiroshi; Shibata, Masahiro; Yui, Mikazu; Neyama, Atsushi*
JNC TN8400 99-079, 287 Pages, 1999/09
Two thermodynamic databases for geochemical calculations supporting research and development on geological disposal concepts for high level radioactive waste are described in this report. One, SPRONS.JNC, is compatible with thermodynamic relations comprising the SUPCRT model and software, which permits calculation of the standard molal and partial molal thermodynamic properties of minerals, gases, aqueous species and reactions from 1 to 5000 bars and 0 to 1000 C. This database includes standard molal Gibbs free energies and enthalpies of formation, standard molal entropies and volumes, and Maier-Kelly heat capacity coefficients at the reference pressure (1 bar) and temperature (25
C. This database includes standard molal Gibbs free energies and enthalpies of formation, standard molal entropies and volumes, and Maier-Kelly heat capacity coefficients at the reference pressure (1 bar) and temperature (25 C) for 195 minerals and 16 gases. It also includes standard partial molal Gibbs free energies and enthalpies of formation, standard partial molal entropies, and Helgeson, Kirkham and Flowers (HKF) equation-of-state coefficients at the reference pressure and temperature for 1147 inorganic and organic aqueous ions and complexes. SPRONS.JNC extends similar databases described elsewhere by incorporating new and revised data published in the peer-reviewed literature since 1991. The other database, PHREEQE.JNC, is compatible with the PHREEQE series of geochemical modeling codes. It includes equilibrium constants at 25
C) for 195 minerals and 16 gases. It also includes standard partial molal Gibbs free energies and enthalpies of formation, standard partial molal entropies, and Helgeson, Kirkham and Flowers (HKF) equation-of-state coefficients at the reference pressure and temperature for 1147 inorganic and organic aqueous ions and complexes. SPRONS.JNC extends similar databases described elsewhere by incorporating new and revised data published in the peer-reviewed literature since 1991. The other database, PHREEQE.JNC, is compatible with the PHREEQE series of geochemical modeling codes. It includes equilibrium constants at 25 C and 1 bar for mineral-dissolution, gas-solubility, aqueous-association and oxidation-reduction reactions. Reaction enthalpies, or coefficients in an empirical log K(T) function, are also included in this database, which permits calculation of equilibrium constants between 0 and 100
C and 1 bar for mineral-dissolution, gas-solubility, aqueous-association and oxidation-reduction reactions. Reaction enthalpies, or coefficients in an empirical log K(T) function, are also included in this database, which permits calculation of equilibrium constants between 0 and 100 C at 1 bar. All equilibrium constants, reaction enthalpies, and logK(T) coefficients in PHREEQE.JNC are calculated usig SUPCRT and SPRONS.JNC, which ensures that these two databases are mutually consistent. They are also internally consistent insofar as all the data are compatible with basic thermodynamic definitions and functional relations in the SUPCRT ...
C at 1 bar. All equilibrium constants, reaction enthalpies, and logK(T) coefficients in PHREEQE.JNC are calculated usig SUPCRT and SPRONS.JNC, which ensures that these two databases are mutually consistent. They are also internally consistent insofar as all the data are compatible with basic thermodynamic definitions and functional relations in the SUPCRT ...
Mochiji, Toshiro; ; Tazaki, Makiko
JNC TN1200 99-002, 44 Pages, 1999/03
The 1999 JNC International Forum on the Peaceful Use of Nuclear Energy the Nuclear Fuel Cycle and Nuclear Non-Proliferation Technology, a continuation of the annual International Forum on Nuclear Non-proliferation sponsored by its predecessor organization PNC (Power Reactor and Nuclear Fuel Development Corporation), was held February 22-23, 1999 in Tokyo, Japan. About 380 people from government, industry, and academia involved in nuclear technology and issues attended the forum. A distinguished group of speakers participated in the forum sessions including highly respected technology and policy experts from France, Russia, and the United States. Session themes were: (1) Advanced Nuclear Technology for Peaceful Purposes and Nuclear Non-Proliferation, (2) Transparency Improvements in Nuclear Technology through the Disposition of Excess Nuclear Weapons Plutonium. This document provides a record of speeches and discussion which have no written documentation and summarizes presented papers as appropriate. Full papers prepared by the presenters can be found in The Proceedings of the 1999 JNC International Forum on the Peaceful Use of Nuclear Energy.
Mochiji, Toshiro; ; Tazaki, Makiko
JNC TN1200 99-001, 20 Pages, 1999/03
no abstracts in English

 and AnO
and AnO
 Species in Geologic Environments
Species in Geologic EnvironmentsChoppin, G. R.*; Bronikowski, M.*; Chen, J.*; Byegard, J.*; Rai, D.*; Yui, Mikazu
JNC TN8400 99-012, 155 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of pentavalent and hexavalent actinide species (AnO and AnO
and AnO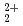 ) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO
) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO and AnO
and AnO
 , redox potentials and equilibrium constants of redox reactions for actinides are also included.
, redox potentials and equilibrium constants of redox reactions for actinides are also included.
Lothenbach, B.*; Ochs, M.*; Wanner, H.*; Yui, Mikazu
JNC TN8400 99-011, 340 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of palladium Pd, lead Pb, tin Sn, antimony Sb, niobium Nb and bismuth Bi in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system of high-level radioactive wastes. Besides treating hydrolysis in detail, this report focuses on the formation of complexes or compounds with chloride, fluoride, carbonate, nitrate, sulfate and phosphate. Other important inorganic ligands (sulfide for lead and antimony, ammonia for palladium) are also included. In this study, the specific ion interaction theory (SIT) approach is used to extrapolate thermodynamic constants to zero ionic strength at 25 C.
C.
Rai, D.*; Rao, L.*; Weger, H. T.*; GREGORY R.CHOPPI*; Yui, Mikazu
JNC TN8400 99-010, 95 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of Pu(III), Am(III), and Cm(III) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. Where data for specific actinide(III) species are lacking, the data were selected based on chemical analogy to other trivalent actinides. In this study, the Pitzer ion-interaction model is mainly used to extrapolate thermodynamic constants to zero ionic strength at 25 C.
C.
Rai, D.*; Rao, L.*; Weger, H. T.*; Felmy, A. R.*; Choppin, G. R.*; Yui, Mikazu
JNC TN8400 99-009, 115 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of Th(IV), U(IV), Np(IV), and Pu(IV) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. Where data for specific actinide(IV) species was lacking, the data were selected based on chemical analogy to other tetravalent actinides. ln this study, the Pitzer ion-interaction model is used to extrapolate thermodynamic constants to zero ionic strength at 25 C.
C.